OSE SERVICES: Verification of Anticorrosion Coatings
Anticorrosion coatings are essential for protecting metallic materials against degradation caused by corrosive environments. OSE SERVICES assists you in verifying and identifying defects in anticorrosion coatings of alloys both on-site and in the laboratory (pre-installation verification, inspection during construction, legal disputes...). We also offer on-site and laboratory services to determine the origin of corrosion.
Different Types of Anticorrosion Coatings
Paints and Organic Coatings
Paints and organic coatings, such as epoxies and polyurethanes, are among the most commonly used. They form a physical barrier that prevents water, salts, and chemicals from penetrating and reacting with the metal. Epoxies offer excellent adhesion and chemical resistance, while polyurethanes are valued for their flexibility and UV resistance.
Metallic Coatings
Metallic coatings include galvanizing (zinc coating) and nickel or chrome plating. Galvanizing is commonly used to protect steel, as zinc preferentially corrodes, thus protecting the underlying metal. Nickel or chrome plating, in addition to providing corrosion protection, offers an aesthetic finish and wear resistance.
Inorganic Coatings
Inorganic coatings, such as zinc silicates, are used for their excellent heat resistance and durability in severe corrosive environments. They are often applied as primers to improve the adhesion of finish layers.
Specialized Polymer Coatings
Coatings based on fluoropolymers (such as PTFE) and polyimides are used for applications requiring exceptional chemical resistance and high thermal stability. These coatings are ideal for aggressive industrial environments.
Anodizing
Anodizing is an electrochemical process primarily used for aluminum. It creates a protective oxide layer that improves corrosion and wear resistance while allowing for colored finishes.
What About Galvanizing?
Galvanizing is a widely used metallic coating process, particularly for protecting steel against corrosion. It involves applying a layer of zinc to the surface of the metal, forming a physical and electrochemical barrier that protects the underlying steel.
Types of Galvanizing
Hot-Dip Galvanizing
This process involves immersing steel in a bath of molten zinc. Hot-dip galvanizing is commonly used for outdoor metal structures such as bridges, utility poles, and agricultural equipment. This type of galvanizing creates a thick and durable coating, providing effective corrosion protection for long periods.
Electrogalvanizing
Also known as electroplating, this process uses an electric current to deposit a thin layer of zinc onto the steel. Electrogalvanizing is often used for applications requiring a smooth and aesthetic finish, such as automotive parts and household appliances. Although the zinc layer is thinner than that obtained by hot-dip galvanizing, it provides sufficient protection in less severe environments.
Advantages of Galvanizing
- Cathodic Protection: Zinc acts as a sacrificial anode. If the zinc layer is damaged, the zinc corrodes preferentially to the steel, offering additional protection.
- Durability: Galvanized coatings can last for several decades, depending on the environment.
- Cost-Effectiveness: Compared to other corrosion protection methods, galvanizing is relatively economical, both in terms of initial costs and long-term maintenance.
Applications of Galvanizing
Galvanizing is used in various sectors, including:
- Construction: Beams, frames, and structural components.
- Infrastructure: Bridges, barriers, and guardrails.
- Automotive Industry: Body parts and chassis.
- Agriculture: Agricultural equipment and fencing.
Galvanizing is a preferred choice for protecting metals due to its robustness, longevity, and ability to provide comprehensive corrosion protection.
Verification of Anticorrosion Coatings, Including Galvanizing
Ensuring the effectiveness and durability of anticorrosion coatings is crucial. Here are the commonly used methods to verify these coatings:
Visual Inspection
Visual inspection is the first step in assessing the condition of coatings. It helps detect surface defects such as cracks, blisters, flaking, and visible corrosion. Inspectors also look for signs of wear or mechanical damage.
Measurement of Coating Thickness
The thickness of the coating is a key indicator of its quality and durability. Several methods can be used:
- Ultrasonic Measurement: Used for thick coatings or when access is limited. It measures thickness by sending ultrasonic waves through the coating.
- Electromagnetic Measurement (Magnetoscope): Used for metallic coatings on ferromagnetic metal substrates. It measures thickness by evaluating the force needed to detach a magnetic probe from the surface.
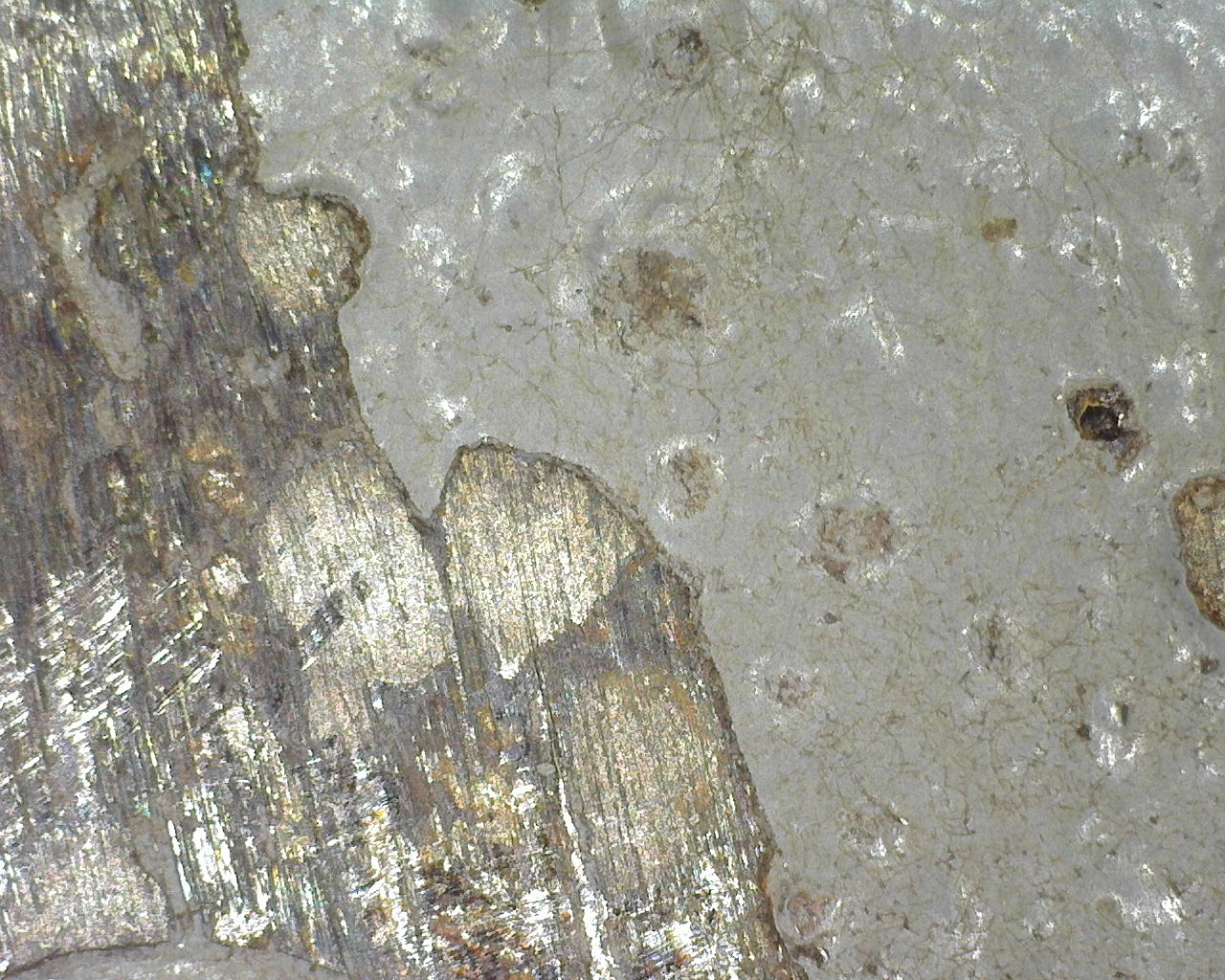
- Boule de Sablage Method (Metallographic Etching): A small section of the coating is removed and examined under a microscope to precisely measure its thickness.
Adhesion Tests
The adhesion of the coating to the substrate is crucial for its performance. Common methods include:
- Cross Hatch Test: A grid pattern is cut into the coating, then adhesive tape is applied and removed to assess the degree of detachment.
- Pull-Off Test: Uses a device to apply a perpendicular pulling force to the coating surface until it detaches, measuring the adhesion strength.
Chemical Composition Analysis
For metallic coatings like galvanizing, chemical analysis can verify the composition and purity of the coating. Techniques such as X-ray fluorescence spectrometry (XRF) can be used to analyze the elemental composition.
Corrosion Resistance Testing
Corrosion resistance tests simulate environmental conditions to evaluate the performance of the coating:
- Salt Spray Test: The coating is exposed to a constant salt mist to simulate corrosion effects. The time before corrosion appears is recorded.
- Acid Fog Test: Similar to the salt spray test but uses an acidic solution for more corrosive environments.
- Climate Cycle Test: Simulates climatic variations to evaluate the coating's resistance to cycles of wet, dry, and saline conditions.
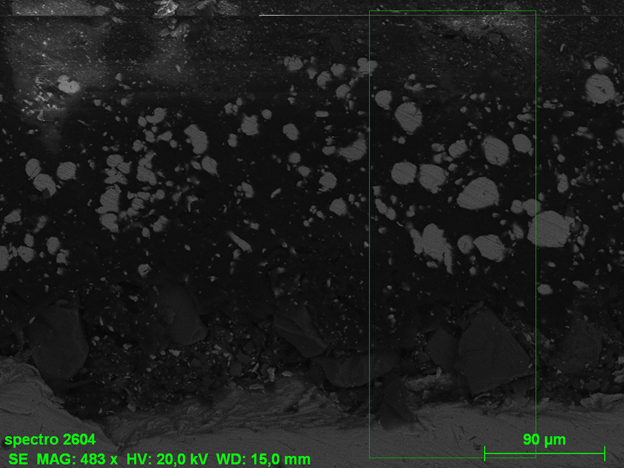
Microscopy
The use of electron microscopes, such as scanning electron microscopy (SEM), allows examining the structure and integrity of the coating at a microscopic scale. This helps identify internal defects and understand the morphology of the coating.
Electrochemical Tests
Electrochemical tests, such as electrochemical impedance spectroscopy (EIS) and corrosion potential measurements, allow evaluating the protective performance of the coating by measuring its electrical properties and behavior in the presence of electrolytes.
By combining these different methods, it is possible to ensure that anticorrosion coatings, including galvanizing, provide effective and durable protection against corrosion.
Contact us for all your expertise requests, on-site interventions, and laboratory analyses.